
The current position:Home > Information dynamic
> Industry Trends
Packaging types and technical requirements of disposable sterile medical devices
source:English website | Release time:2022年09月07日As a medical device product, the most basic requirement is to be safe and effective and to ensure human health and life safety.
As the last process of disposable medical devices (except sterilization), the processing level of packaging plays a decisive role in the quality of the whole product. Therefore, improving the processing and manufacturing level of packaging plays an irreplaceable role in improving and ensuring the safety of medical devices. Throughout the history of the packaging production of disposable sterile medical devices in China, like most foreign enterprises, most of them developed from food packaging bag processing enterprises, which always has the problem of low starting point and slow development. However, in recent years, with the increasing supervision of the disposable sterile medical device industry in China and the entry of foreign-funded enterprises, great progress has been made in terms of environment purification, processing equipment, material level, test means, processing technology and technology level.
Nevertheless, at this stage, the overall level of domestic processing enterprises, whether in processing, technology or testing, still lags behind that of foreign enterprises.
The above is the packaging types and technical requirements of disposable sterile medical devices summarized by Changshu Boyi Medical Devices Co., Ltd. for you. If you still have any relevant knowledge, please consult us
Prev:
Introduction to disposable sterile medical devices
Next:
Development and market scale analysis of disposab…

 Cn
Cn En
En WeChat ID:
WeChat ID: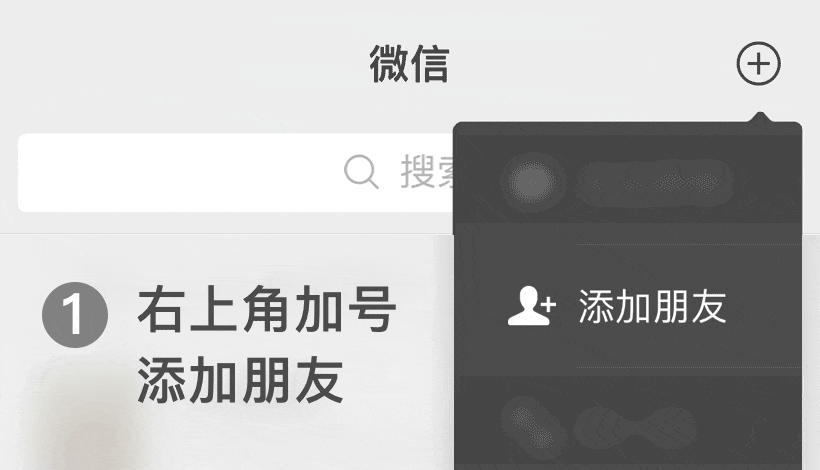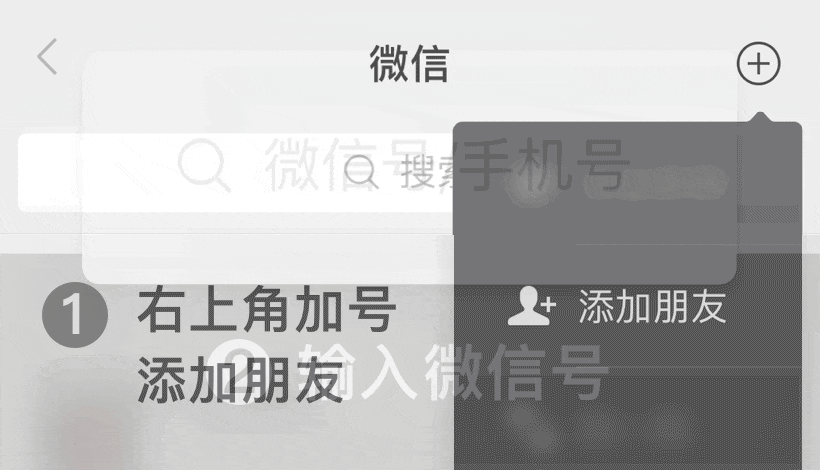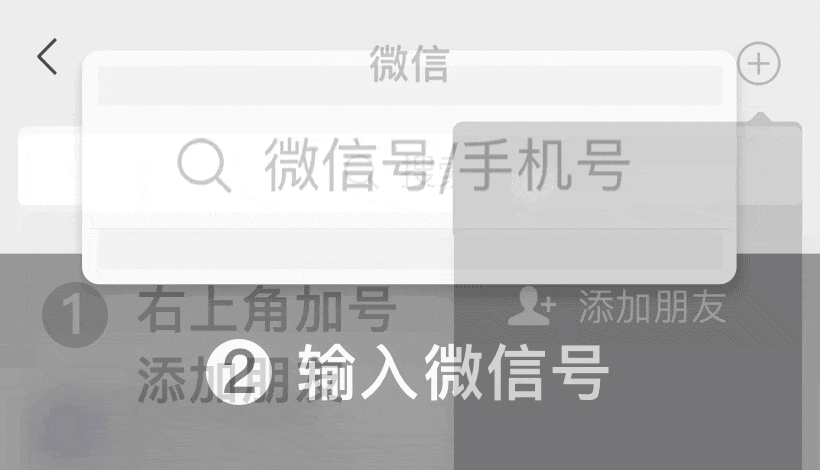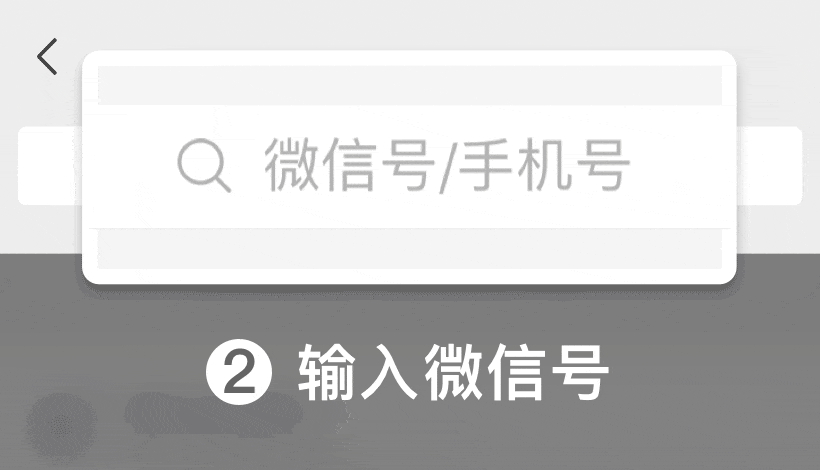




 Contact us
Contact us
 Add WeChat
Add WeChat
 Telephone
Telephone